Atovaquone, a drug used to treat malaria, is known to inhibit the mitochondrial electronic transport chain. In this study, conducted in France by researchers from the Institut de chimie radicalaire (CNRS / Aix-Marseille Université), a new agent derived from atovaquone targeting mitochondria (Mito-ATO) was developed to prevent tumor growth and spread. This research work has been published in the journal Advanced Science.
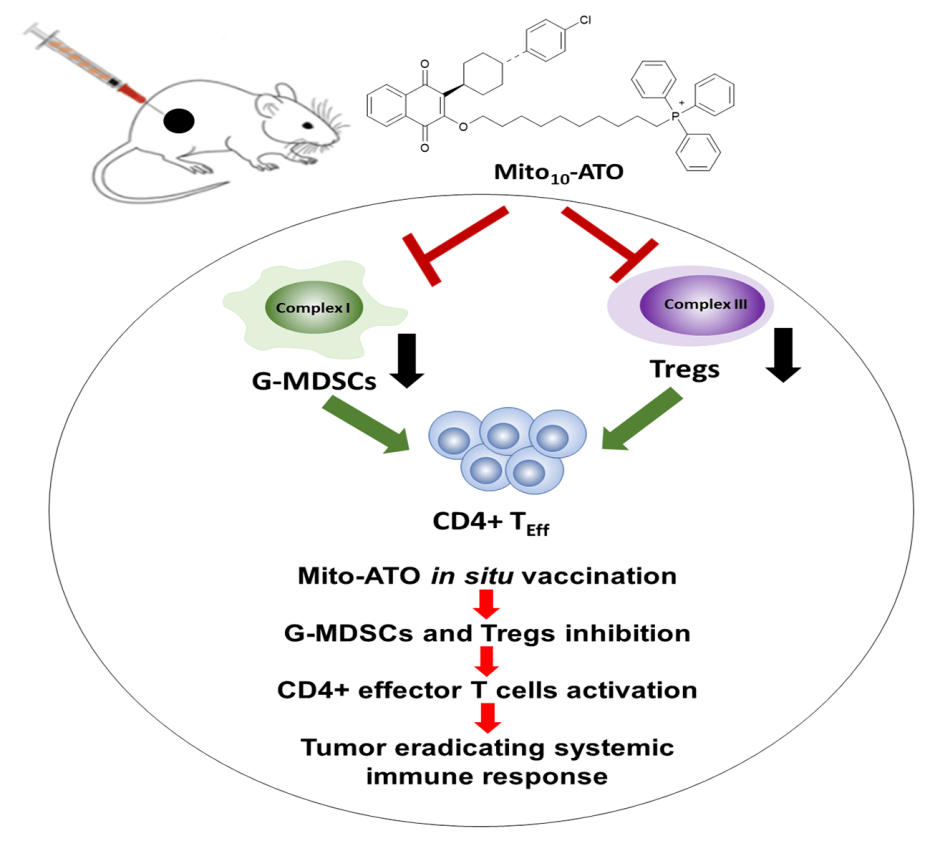
Mito-ATO vectorization increases its accumulation in the mitochondria of cancer cells, as well as in immunosuppressive cells within the tumor immune microenvironment (TIME). These populations of so-called immunosuppressive cells, such as myeloid-derived suppressor cells (g-MDSCs) and regulatory T cells (Tregs), are amplified in pathological situations such as cancer, hence the need to reduce their numbers.
Using an in situ vaccination approach, injection of Mito-ATO into primary tumors triggered potent T-cell immune responses locally, but also at distant tumor sites. As demonstrated in transplantable tumor models and in a spontaneous tumor model, this immune response also leads to the attack of tumor cells in localized foci in different parts of the body. In situ vaccination with Mito-ATO prevents the spread of lung cancer metastases and their development in the brain. Furthermore, flow cytometry analysis revealed that Mito-ATO treatment leads to a significant reduction of g-MDSCs but also of Tregs lymphocytes within TIME.
Single-cell transcriptomics (scRNA-seq) showed that Mito-ATO treatment blocks the expression of genes responsible for oxidative phosphorylation (OXPHOS) and glycolysis in g-MDSCs and Tregs cells. This would lead to cell death of these cells in the tumor microenvironment, which explains the decrease observed with Mito-ATO treatment.
Mito-ATO inhibits the expression of complex I, V, OXPHOS and glycolysis genes in g-MDSCs cells, and the expression of complex I, III, IV, OXPHOS and glycolysis genes in Tregs cells. The intratumoral decrease of g-MDSCs and Tregs cells could facilitate the observed increase in tumor-infiltrating CD4+ T cells. These results highlight the antitumor efficacy of Mito-ATO on new targets (g-MDSCs and Tregs) of TIME.
The strategy of targeting g-MDSCs and Tregs with Mito-ATO is thus an innovative and attractive approach to regulate tumor immunity in order to effectively prevent and treat cancers and their metastasis.
Read more: Huang, M., Xiong, D., Pan, J., Zhang, Q., Wang, Y., Myers, C. R., Johnson, B. D., Hardy, M., Kalyanaraman, B., You, M., Prevention of Tumor Growth and Dissemination by In Situ Vaccination with Mitochondria-Targeted Atovaquone. Adv. Sci. 2022, 2101267. https://doi.org/10.1002/advs.202101267
This research was conducted by French researchers from the Institut de chimie radicalaire (CNRS/Aix-Marseille Université) in collaboration with American researchers from the Medical College of Wisconsin and the Houston Methodist Research Institute.
Researcher contact:
Institute of Radical Chemistry
Micael Hardy - micael.hardy[at]univ-amu.fr